Abstract
In AML, nucleophosmin (NPM1) is the gene that is most frequently mutated. Mutated NPM1 (ΔNPM1) is a driver gene that is essential for malignant transformation early in leukemogenesis. ΔNPM1 arises from a 4 base pair (4-bp) frameshift insertion in exon 12, resulting in a protein that is 4 amino acids (AA) longer with its C-terminal 11 AA translated in a novel alternative reading frame (CLAVEEVSLRK). The recurrent frameshift insertion occurs at restricted positions in the coding sequence and although the exact 4-bp sequence can differ, the majority of mutations encode the same alternative reading frame. Since ΔNPM1 is a clonal driver mutation in 30% of AML that persists during disease relapse, its novel alternative reading frame is an attractive target for immunotherapy.
To investigate whether ΔNPM1 peptides are processed and presented on AML, we searched in the HLA class I ligandome of 12 primary AML for ligands matching a protein region spanning 10 N-terminal AA in the normal reading frame followed by the 11 C-terminal AA in the alternative reading frame (MTDQEAIQDL CLAVEEVSLRK). This revealed the presence of 5 peptides (VEEVSLRK, AVEEVSLR, CLAVEEVSL, AVEEVSLRK and CLAVEEVSLRK) in AML with ΔNPM1, but not in AML with wild type NPM1 (wtNPM1). All 5 ligands as eluted from 7 of 8 AML with ΔNPM1 were validated by comparing tandem mass spectra with synthetic peptides. The eluted CLAVEEVSL and CLAVEEVSLRK peptides were validated upon cysteinylation of the first residue of the synthetic peptide. Online algorithms predicted binding of CLAVEEVSL to HLA-A*02:01 and binding of both AVEEVSLRK and CLAVEEVSLRK to HLA-A*03:01 as well as -A*11:01, which was confirmed by appropriate folding of the respective HLA class I monomers in vitro .
Since HLA-A*02:01 is expressed in 50% of the Caucasian population, we selected CLAVEEVSL to explore its relevance as therapeutic neoantigen and searched for specific T cells in patients with AML. Peptide-MHC tetramers were produced for CLAVEEVSL and its cysteinylated variant C*LAVEEVSL and a mix of tetramers was used to isolate specific T cells from PBMC from 6 HLA-A*02:01 positive patients with ΔNPM1 AML who were in remission after chemotherapy. No specific T cells could be isolated, indicating that T cells for ΔNPM1 did not exist at frequencies that could be detected in limited numbers of PBMC from these AML patients. We subsequently screened large numbers of PBMC from 6 HLA-A*02:01 positive healthy individuals and isolated various tetramer positive T cell clones. One of these clones, clone 1A2, specifically reacted against HLA-A*02:01 positive AML with ΔNPM1, but lacked reactivity against AML with wtNPM1 in IFN-γ ELISA. Clone 1A2 also lacked reactivity against HLA-A*02:01 negative AML with ΔNPM1 as well as EBV-transformed B lymphoblastoid cell lines and monocyte-derived mature dendritic cells from HLA-A*02:01 positive AML patients.
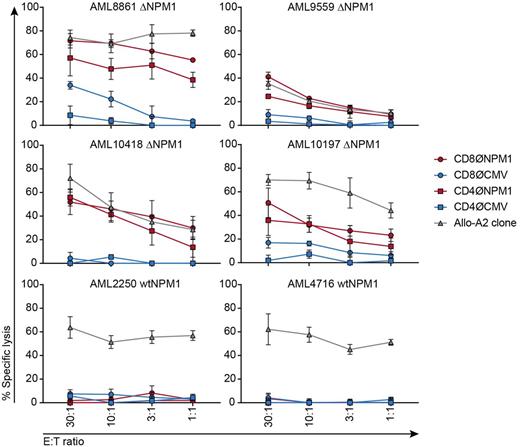
To investigate CLAVEEVSL as target for T cell receptor (TCR) gene transfer, the variable regions of the TCR α and β chains from clone 1A2 were sequenced, synthesized and cloned into the MP71-TCR-flex retroviral vector. The TCR for ΔNPM1 and, as a control, the TCR for HLA-A*02:01-restricted CMV peptide NLVPMVATV, were introduced in CD8 and CD4 cells that were isolated from healthy HLA-A*02:01 positive individuals. TCR-transduced cells were tested for anti-tumor reactivity against a panel of 13 HLA-A*02:01 positive AML in IFN-γ ELISA. Upon transduction with the TCR for ΔNPM1, CD8 and CD4 cells both showed recognition of all 9 AML with ΔNPM1, but not 4 AML with wtNPM1. ΔNPM1 TCR-transduced cells also failed to recognize HLA-A*02:01 negative AML and HLA-A*02:01 positive autologous monocyte-derived mature dendritic cells. Next, we tested the cytolytic capacity of TCR-transduced cells against HLA-A*02:01 positive AML in a 9 hrs 51chromium release assay. Both CD8 and CD4 cells transduced with the TCR for ΔNPM1 showed specific lysis of all 4 AML with ΔNPM1 tested, but not 2 AML with wtNPM1 (Fig. 1).
In conclusion, our results show that various ΔNPM1-derived peptides are presented on the surface of primary AML and that CLAVEEVSL is a neoantigen that can be efficiently targeted by TCR gene transfer in a co-receptor independent fashion. Immunotherapy targeting ΔNPM1 may therefore contribute to treatment of patients with AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal